来源:研之成理
▲第一作者:佟月宇;通讯作者:梁骥,周思,刘健 通讯单位: 天津大学/伍伦贡大学(梁骥)、大连理工大学/伍伦贡大学(周思)、大连化学物理研究所/大连-萨里未来材料研究中心(刘健)论文DOI:10.1002/anie.202002029全文速览缺陷工程铁掺杂W18O49纳米反应器在-0.15V (相对于可逆氢电极)的低电位下同时获得了24.7 μg h-1 mg-1cat.的NH3产率和20.0%的法拉第效率。

背景介绍
NH3是现代工业和农业生产最为基础的化工原料之一,与人类的生产、生活息息相关,不可或缺。尽管大量研究报道了氧空位缺陷的引入与外来原子掺杂的共同作用可有效固氮(NRR),但是NRR的主要竞争析氢反应(HER)及其自身极高的常温电化学反应能垒,使得在低电位下,同时获得高的氨产量和法拉第效率成为亟待攻克的难题。
研究出发点选用对HER具有极低选择性的W18O49为调控对象,通过一步法获得Fe掺杂的生长在碳纤维纸上的W18O49。结果发现,在0.25 M LiClO4溶液中,该催化剂在-0.15V (相对于可逆氢电极)的低电位下同时获得了24.7 μg h-1 mg-1cat.的NH3产率和20.0%的法拉第效率,优于目前已报道的绝大多数水溶液电解质环境工作的电化学合成氨催化剂。
图文解析
与传统的哈伯-博世法固氮相比,室温下的电催化氮气还原过程由于具有高能效、低排放等优点逐步吸引了越来越多研究者们的兴趣。澳大利亚伍伦贡大学梁骥课题组长期致力于用于电催化剂与电催化过程的设计和研发。刘健团队长期致力于纳米反应器与反应工程研究,两个团队合作,通过一步法(Figure 1)缺陷工程铁掺杂策略很好的控制了W18O49中的氧空位状态。
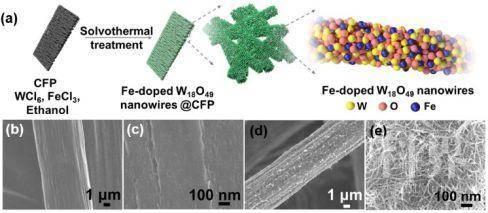 ▲Figure 1. (a) Schematic illustration of the preparation of Fe-doped W18O49 nanowires @ CFP. SEM images of (b-c) CFP, (d-e) W18O19-16Fe nanowires @ CFP.
▲Figure 1. (a) Schematic illustration of the preparation of Fe-doped W18O49 nanowires @ CFP. SEM images of (b-c) CFP, (d-e) W18O19-16Fe nanowires @ CFP.
结合周思带领的理论计算团队,通过Ab initio计算我们进一步发现,利用W18O49固有低的氢气结合能、缺陷工程调控得到的最大化的活性位点(W)的暴露,极大地削弱了与固氮竞争的析氢反应,并降低了室温下电化学氮还原反应的能垒。因此,在相对可逆氢电极-0.15 V的极低电位下,优化铁掺量的W18O49同时获得了高的氨产率(24.7 μg h−1 )和高的法拉第效率(20.0%)(Figure 2)。在电催化过程中,该催化剂同样表现出良好的稳定性。
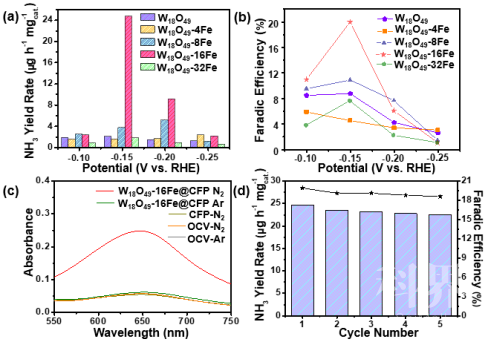 ▲Figure 2. (a) NH3 yield rate at various potentials vs. RHE. (b) Faradic efficiency of as-prepared Fe-doped W18O49 nanowires @ CFP electrodes. (c) UV-vis absorption spectra of various electrolytes stained with salicylic acid indicator. OCV stands for open-circuit voltage. (d) cycling stability results at −0.15 V (vs. RHE) of W18O49-16Fe nanowires @ CFP electrode.
▲Figure 2. (a) NH3 yield rate at various potentials vs. RHE. (b) Faradic efficiency of as-prepared Fe-doped W18O49 nanowires @ CFP electrodes. (c) UV-vis absorption spectra of various electrolytes stained with salicylic acid indicator. OCV stands for open-circuit voltage. (d) cycling stability results at −0.15 V (vs. RHE) of W18O49-16Fe nanowires @ CFP electrode.
Ab initio计算(Figure 3)进一步揭示了我们提出的铁的嵌入掺杂策略和调整后的氧空位状态同时优化了W18O49的电子状态和表面结构,从而带来提高的氮气吸附强度,并获得低电位的反应路径。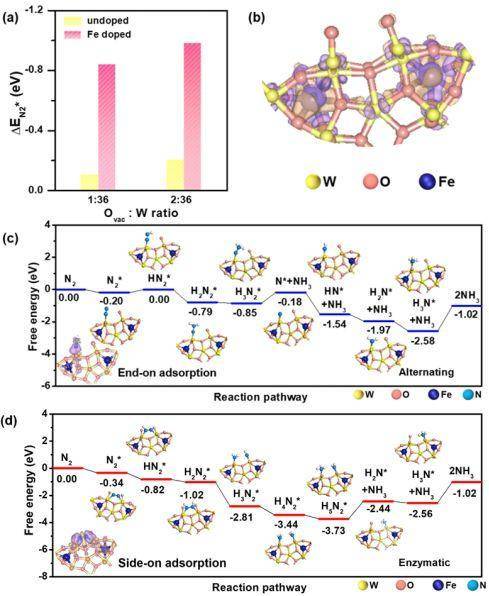 ▲Figure 3. (a) N2 adsorption energy (ΔEN2*) as a function of Ovac : W ratio for W18O49 with and without Fe doping by DFT calculations. (b) The differential charge density between Fe and W18O49 with Ovac : W ratio of 2 : 36 without N2 chemisorption. Purple and orange colors represent electron accumulation and depletion regions, respectively, with isosurface value of 5×10−3 eÅ−3. (c, d) Free energy diagrams of NRR on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The structures of reaction intermediates are shown next to their energy segments. Left bottom of (c, d) inserted the differential charge density distributions of N2 chemisorbed on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The Fe/W ratio is fixed at 11%.
▲Figure 3. (a) N2 adsorption energy (ΔEN2*) as a function of Ovac : W ratio for W18O49 with and without Fe doping by DFT calculations. (b) The differential charge density between Fe and W18O49 with Ovac : W ratio of 2 : 36 without N2 chemisorption. Purple and orange colors represent electron accumulation and depletion regions, respectively, with isosurface value of 5×10−3 eÅ−3. (c, d) Free energy diagrams of NRR on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The structures of reaction intermediates are shown next to their energy segments. Left bottom of (c, d) inserted the differential charge density distributions of N2 chemisorbed on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The Fe/W ratio is fixed at 11%.
总结与展望此工作不仅为常温电化学固氮提供了一种高活性的非贵金属催化剂,而且为各种电催化过程提供了一个合理的催化剂设计新思路。
 ▲Figure 1. (a) Schematic illustration of the preparation of Fe-doped W18O49 nanowires @ CFP. SEM images of (b-c) CFP, (d-e) W18O19-16Fe nanowires @ CFP.
▲Figure 1. (a) Schematic illustration of the preparation of Fe-doped W18O49 nanowires @ CFP. SEM images of (b-c) CFP, (d-e) W18O19-16Fe nanowires @ CFP.结合周思带领的理论计算团队,通过Ab initio计算我们进一步发现,利用W18O49固有低的氢气结合能、缺陷工程调控得到的最大化的活性位点(W)的暴露,极大地削弱了与固氮竞争的析氢反应,并降低了室温下电化学氮还原反应的能垒。因此,在相对可逆氢电极-0.15 V的极低电位下,优化铁掺量的W18O49同时获得了高的氨产率(24.7 μg h−1 )和高的法拉第效率(20.0%)(Figure 2)。在电催化过程中,该催化剂同样表现出良好的稳定性。
 ▲Figure 2. (a) NH3 yield rate at various potentials vs. RHE. (b) Faradic efficiency of as-prepared Fe-doped W18O49 nanowires @ CFP electrodes. (c) UV-vis absorption spectra of various electrolytes stained with salicylic acid indicator. OCV stands for open-circuit voltage. (d) cycling stability results at −0.15 V (vs. RHE) of W18O49-16Fe nanowires @ CFP electrode.
▲Figure 2. (a) NH3 yield rate at various potentials vs. RHE. (b) Faradic efficiency of as-prepared Fe-doped W18O49 nanowires @ CFP electrodes. (c) UV-vis absorption spectra of various electrolytes stained with salicylic acid indicator. OCV stands for open-circuit voltage. (d) cycling stability results at −0.15 V (vs. RHE) of W18O49-16Fe nanowires @ CFP electrode.Ab initio计算(Figure 3)进一步揭示了我们提出的铁的嵌入掺杂策略和调整后的氧空位状态同时优化了W18O49的电子状态和表面结构,从而带来提高的氮气吸附强度,并获得低电位的反应路径。
 ▲Figure 3. (a) N2 adsorption energy (ΔEN2*) as a function of Ovac : W ratio for W18O49 with and without Fe doping by DFT calculations. (b) The differential charge density between Fe and W18O49 with Ovac : W ratio of 2 : 36 without N2 chemisorption. Purple and orange colors represent electron accumulation and depletion regions, respectively, with isosurface value of 5×10−3 eÅ−3. (c, d) Free energy diagrams of NRR on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The structures of reaction intermediates are shown next to their energy segments. Left bottom of (c, d) inserted the differential charge density distributions of N2 chemisorbed on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The Fe/W ratio is fixed at 11%.
▲Figure 3. (a) N2 adsorption energy (ΔEN2*) as a function of Ovac : W ratio for W18O49 with and without Fe doping by DFT calculations. (b) The differential charge density between Fe and W18O49 with Ovac : W ratio of 2 : 36 without N2 chemisorption. Purple and orange colors represent electron accumulation and depletion regions, respectively, with isosurface value of 5×10−3 eÅ−3. (c, d) Free energy diagrams of NRR on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The structures of reaction intermediates are shown next to their energy segments. Left bottom of (c, d) inserted the differential charge density distributions of N2 chemisorbed on Fe-doped W18O49 with Ovac : W ratio of 1 : 36 and 2 : 36, respectively. The Fe/W ratio is fixed at 11%.总结与展望此工作不仅为常温电化学固氮提供了一种高活性的非贵金属催化剂,而且为各种电催化过程提供了一个合理的催化剂设计新思路。
参考文献[1] Hao Tian, Ji Liang and Jian Liu, Nanoengineering carbon spheres as nanoreactors for sustainable energy applications. Advanced Materials, 2019, 31, 1903886.[2] Tianyu Yang, Lijuan Wei, Lingyan Jing, Jifen Liang, Xiaoming Zhang, Min Tang, Michael J. Monteiro, Ying (Ian) Chen, Yong Wang, Sai Gu, Dongyuan Zhao, Hengquan Yang, Jian Liu and G. Q. Max Lu, Dumbbell-shaped bi-component mesoporous Janus solid nanoparticles for biphasic interface catalysis. Angewandte Chemie International Edition, 2017, 56, 8459-8463.[3] Yash Boyjoo, Meiwen Wang, Vishnu K. Pareek, Jian Liu and Mietek Jaroniec, Synthesis and Applications of Porous Non-silica Metal Oxide Submicrospheres. Chemical Society Reviews, 2016, 45, 6013-6047.[4] Y. Y. Tong, X. Yan, J. Liang, S. X. Dou, Metal‐Based Electrocatalysts for Methanol Electro‐Oxidation: Progress, Opportunities, and Challenges. Small, 2019, 1904126.[5] X. Yan, D. L. Liu, H. H. Cao, F. Hou, J. Liang, S. X. Dou, Nitrogen Reduction to Ammonia on Atomic‐Scale Active Sites under Mild Conditions. Small Methods, 2019, 3, 1800501.[6] S. H. Wang, X. Yan, K. H. Wu, X. M. Chen, J. M. Feng, P. Y. Lu, F. Hou, H. M. Cheng, J. Liang, S. X. Dou, A hierarchical porous Fe-N impregnated carbon-graphene hybrid for high-performance oxygen reduction reaction. Carbon, 2019, 144, 798-804.[7] X. D. Hong, R. Wang, Y. Liu, J. W. Fu, J. Liang, S. X. Dou, Recent advances in chemical adsorption and catalytic conversion materials for Li–S batteries. Journal of Energy Chemistry, 2020, 42, 144-168.[8] J. Mei, T. Liao, J. Liang, Y. Qiao, S. X. Dou, Z. Sun, Toward Promising Cathode Catalysts for Nonlithium Metal-Oxygen Batteries. Advanced Energy Materials, 2019, 1901997.[9] D. L. Liu, Y. Y. Tong, X. Yan, J. Liang, S. X. Dou, Batteries & Supercaps, 2019, 2, 743.[10] J Liang, L. Yin, X. Tang, H. Yang, W. Yan, L. Song, H. M. Cheng, F. Li, Kinetically enhanced electrochemical redox of polysulfides on polymeric carbon nitrides for improved lithium–sulfur batteries. ACS applied materials & interfaces, 2016, 8 (38), 25193-25201.
来源:rationalscience 研之成理
原文链接:https://mp.weixin.qq.com/s?__biz=MzIwMzE5MzQ1NQ==&mid=2649343924&idx=4&sn=4a659f9dc532b6e4e0e656d72eea4e69&chksm=8ece4ab4b9b9c3a2d45e18c56ef8b7c24b80617bb2481b157f49a29e76cfd9885f617cecc0a0#rd
版权声明:除非特别注明,本站所载内容来源于互联网、微信公众号等公开渠道,不代表本站观点,仅供参考、交流、公益传播之目的。转载的稿件版权归原作者或机构所有,如有侵权,请联系删除。
电话:(010)86409582
邮箱:kejie@scimall.org.cn



